Meet the Experts coupled plasma
The Role of Inductively Coupled
Plasma-Optical Emission Spectrometry
in Determining the Fineness of
Precious Metals
Accurate precious metal analysis is routinely carried out to determine the precious metal content in a wide variety of different materials. This is particularly important for fineness determination for the hallmarking of precious metals for jewellery use (see Figure 1). The precious metal concentration in different materials can vary from parts per million (ppm) or lower in car catalyst or powdered ore samples, to virtually 100% in jewellery alloys or bullion bars. This article considers the benefits of analysis by inductively coupled plasma-optical emission spectrometry (ICP-OES).
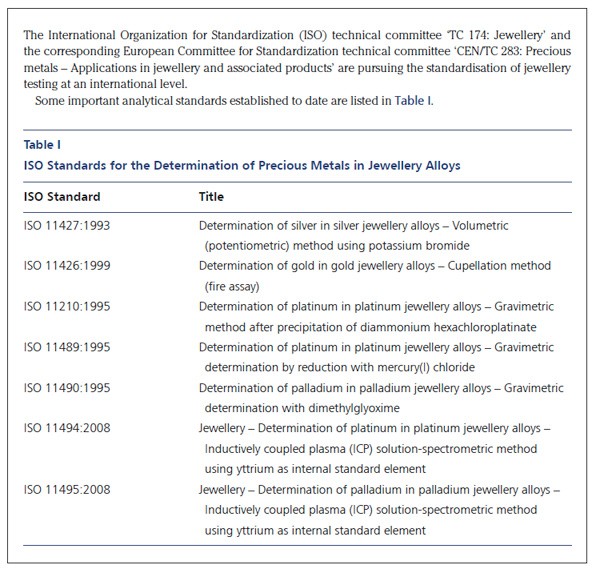
The wide range of sample types in which precious metals occur require that a variety of classical and instrumental methods, or combinations of the two, be used in precious metal testing. In the last six decades more than 400 methods have been published for the analysis of precious metals (1, 2). Even an experienced analytical chemist may hesitate to choose the most suitable procedure for separation and determination of precious metals in a material of unknown composition. There can be a temptation to accept the more recently published procedure as being superior to those which are well tried and tested.
Analysing Precious Metal Content
It is estimated that less than 10% of all precious metal determinations can be performed by strictly instrumental techniques. Probably no more than 30% of all determinations are carried out by strictly classical chemical methods. Hence a large portion of the analytical workload must be handled by a combination of chemical and instrumental approaches, which as a result become strongly interdependent. Fire assay and smelting based techniques (Figure 2) are the earliest analytical procedures recorded in human history, and remain essential pretreatment steps for a large variety of samples.
To determine whether an analytical method can achieve the required performance for fi neness determination, the method must be tested by frequent repetition, ideally with the same sample.
Inductively Coupled Plasma-Optical Emission Spectrometry
Assay Office Birmingham uses ICP-OES extensively. It is one of the best techniques available for the analysis of the platinum group metals (pgms): platinum, palladium, rhodium, iridium and ruthenium, as well as base metals such as copper, zinc and nickel, being accurate to 1 ppm. The technique is destructive but it does accurately determine both major and trace elements.
Using this technique, a small sample is accurately weighed and dissolved in an appropriate acidic matrix (Figure 3) then subjected to temperatures (plasma) high enough to cause excitation of the sample atoms. Once the atoms or ions are in their excited states, they decay to lower stable states by releasing energy. Light is emitted at a specifi c wavelength by each element present and these emission lines are measured and used to determine the concentrations of the elements of interest.
The high precision of ICP methods is due to the stability of intensity quotients of emission lines, essentially of gold (267.6 nm), platinum (265.9 nm), palladium (340.5 nm) and the internal standard element yttrium (371.02 nm). If the precious metal and yttrium lines are measured simultaneously, a reproducibility in results of at least 0.01% can be achieved.
Comparison with Classical Methods
Classical methods such as gravimetry have maintained their importance and set the standard for the accuracy of instrumental methods. In order to be approved by the ISO as an alternative analytical technique for determining the fineness of precious metal alloys, ICP methods would be required to achieve at least the same level of repeatability as gravimetric methods. In addition it is especially crucial that interference-free direct measurement of the precious metal can be achieved. This is, of course, necessary for the optimisation of ICP-OES. Over a period of time it has been recognised that
ICP-OES can be applied for a wide variety of precious metal alloys. The method is more easily and economically carried out than gravimetry, and can also be faster, as it can be used for multiple element determinations at the same time. It gives results with acceptable precision and its chemical selectivity is higher than for gravimetry. Above all, it attains a reproducibility and accuracy comparable with those of gravimetry.
Conclusion
ICP-OES is now routinely employed by several commercial laboratories for the analysis of pgm-containing powder and ore samples, after fi re assay with nickel sulfi de as the collector. It is common practice for the analysis of some non-metallic materials, such as car catalyst samples, to use a combination of both chemical (gravimetric) and instrumental approaches.
The precision and accuracy of ICP methods make them highly suitable for fi neness determination of precious metals for hallmarking. Anybody interested in the analytical chemistry of precious metals would do well to study the excellent reference works by Beamish and Van Loon (1), Beamish (3), Bugbee (4) and Smith (5, 6).
References
1 F. E. Beamish and J. C. Van Loon, “Recent Advances in the Analytical Chemistry of the Noble Metals”, Pergamon Press, Oxford, 1972
2 F. E. Beamish, J. C. Van Loon and C. L. Lewis, “Analysis of Noble Metals: Overview and Selected Methods”,
Academic Press, New York, USA, 1977 3 F. E. Beamish, “The Analytical Chemistry of the Noble
Metals”, Pergamon Press, Oxford, UK, 1966
4 E. E. Bugbee, “A Textbook of Fire Assaying”, Chapman and Hall, New York, USA, 1940
5 E. A. Smith, “The Sampling and Assay of the Precious Metals: Comprising Gold, Silver, Platinum, and the Platinum Group Metals in Ores, Bullion, and Products”,
2nd Edn., C. Griffi n, London, UK, 1947
6 E. A. Smith, “Working in Precious Metals”, 2nd Revised Edn., NAG Press, London, UK, 1978
Your item has been added to the basket
You need to create an account, or login before you can add this item to your basket.

 Dippal Manchanda is the chief assayer and technical director at Assay Office Birmingham, responsible for maintaining high analytical standards and providing scientific and technical expertise to all divisions of the business. Dippal holds a Masters degree (MSc) in inorganic chemistry and has over 20 years of experience in assaying and the examination of precious metals and alloys. He is also a Fellow of the Royal Society of Chemistry and has attained the level of membership of ‘Chartered Chemist’. The UK Science Council has awarded him the status of ‘Chartered Scientist’ – a recognition awarded to those scientists who demonstrate the application to stay up to date in their field. During his varied career, Dippal has been involved in several prestigious precious metal projects including setting up a state-of-the-art gold/silver medallion manufacturing facility for a public sector undertaking in India.
Dippal Manchanda is the chief assayer and technical director at Assay Office Birmingham, responsible for maintaining high analytical standards and providing scientific and technical expertise to all divisions of the business. Dippal holds a Masters degree (MSc) in inorganic chemistry and has over 20 years of experience in assaying and the examination of precious metals and alloys. He is also a Fellow of the Royal Society of Chemistry and has attained the level of membership of ‘Chartered Chemist’. The UK Science Council has awarded him the status of ‘Chartered Scientist’ – a recognition awarded to those scientists who demonstrate the application to stay up to date in their field. During his varied career, Dippal has been involved in several prestigious precious metal projects including setting up a state-of-the-art gold/silver medallion manufacturing facility for a public sector undertaking in India.